Drug rash and disease progression in MF and SS can look very similar1,a
Skin biopsy is recommended for differential diagnosis1
Peripheral blood flow cytometry and skin biopsy with T-cell receptor sequencing should also be considered.2
Drug rash
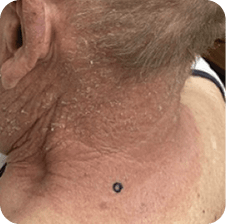
Neck
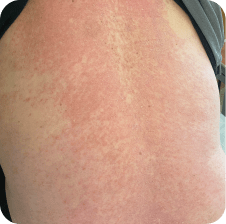
Back
Skin lesions in MF and SS
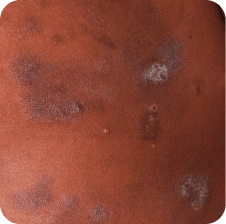
Torso
Torso
Which image shows a case of drug rash vs skin lesions in MF and SS?
Click the images below to reveal the answer
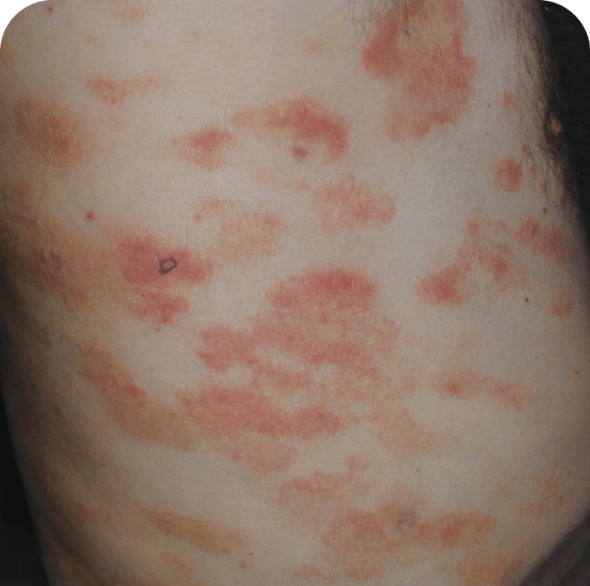
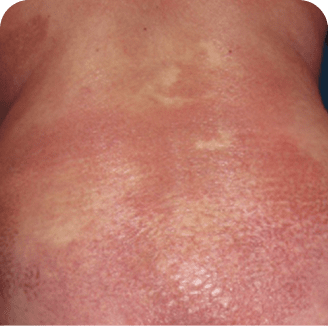
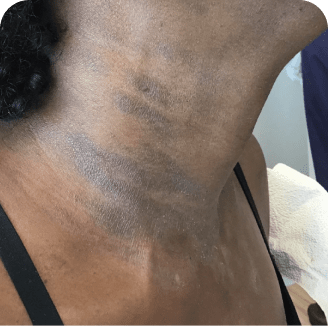

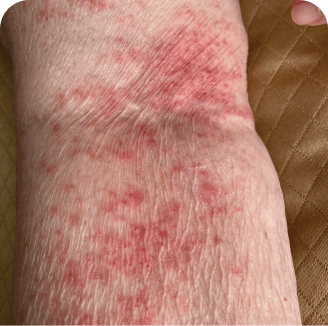

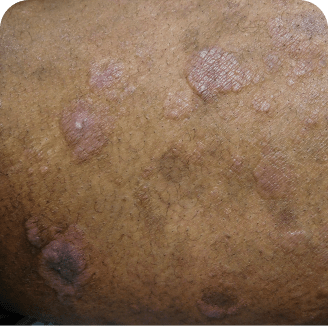

Photographs are intended as examples. Actual presentation can vary depending on individual patient factors.
Photos courtesy of Oleg Akilov, MD, PhD, Joan Guitart, MD, and Lauren Hughey, MD.
Steps to differentiate skin lesions
Monitor patients for rash throughout the treatment course1
Onset of drug rash is variable. In the MAVORIC trial, median time to onset was 15 weeks; 25% of cases occurred after 31 weeks.
Perform a physical exam and review of symptoms
Check for noncutaneous signs/symptoms of disease progression such as enlarged lymph nodes, fever, chills, weight loss, or fatigue.3
Note the appearance of new lesions
Most common presentation of drug rash in MAVORIC1,a:
- Papular or maculopapular rash
- Lichenoid rash
- Morbilliform rash
- Spongiotic or granulomatous dermatitis
Consider a skin biopsy1
Histology, IHC, and clonality studies have been shown to be helpful in distinguishing drug rash from disease progression. Specifically, biopsy results showing exocytosis of CD8-positive T cells, a normal or inverted CD4:CD8 positive ratio, retention of CD7 expression, and polyclonality of TCR-β and TCR-γ are generally consistent with drug rash.4-6
Dermatopathology consult may provide definitive diagnosis and help determine treatment path2,7
Download this tool to help differentiate skin lesions from cases of drug rash
Real-world evidence indicates that SS patients are more likely to
develop drug rash than MF patients8

Incidence of rash, including drug rash1,a
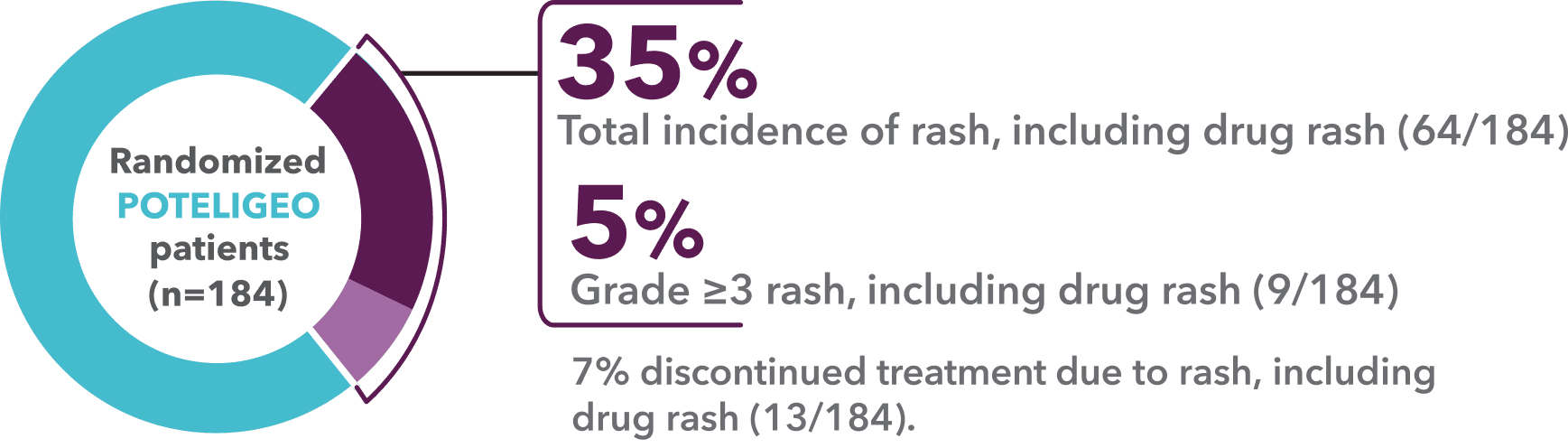
- “Rash, including drug rash” was the most common adverse reaction in MAVORIC (35%)1,9
- The incidence of drug rash alone was 24%1,9
- Once drug rash was resolved, a majority of patients appeared to have resumed treatment10,b
- aDrug rash may also be referred to as drug eruption.
- bBased on a subanalysis of 44 of the 64 patients who experienced drug rash in MAVORIC in which 35 (80%) resumed treatment after resolution of initial drug rash per the study’s protocol.1,10
- IHC=immunohistochemistry; MF=Mycosis Fungoides; SS=Sézary Syndrome; TCR=T-cell receptor.
POTELIGEO is administered in 28-day cycles1
- POTELIGEO [package insert]. Kyowa Kirin Inc., Princeton, NJ USA.
- Chen L, Carson KR, Staser KW, et al. Mogamulizumab-associated cutaneous granulomatous drug eruption mimicking mycosis fungoides but possibly indicating durable clinical response. JAMA Dermatol. 2019;155(8):968-971.
- Ludmann P. Cutaneous T-cell lymphoma: symptoms. American Academy of Dermatology Association. Updated August 3, 2023. Accessed January 24, 2025. https://www.aad.org/public/diseases/a-z/ctcl-symptoms
- Hirotsu KE, Neal TM, Khodadoust MS, et al. Clinical characterization of mogamulizumab-associated rash during treatment of mycosis fungoides or Sézary syndrome. JAMA Dermatol. 2021;157(6):700-707.
- Trum NA, Zain J, Martinez XU, et al. Mogamulizumab efficacy is underscored by its associated rash that mimics cutaneous T-cell lymphoma: a retrospective single-centre case series. Br J Dermatol. 2022;186(1):153-166.
- Wang JY, Hirotsu KE, Neal TM, et al. Histopathologic characterization of mogamulizumab-associated rash. Am J Surg Pathol. 2020;44(12):1666-1676.
- Poligone B, Querfeld C. Management of advanced cutaneous T-cell lymphoma: role of the dermatologist in the multidisciplinary team. Br J Dermatol. 2015;173(4):1081-1083.
- Assaf C, Booken N, Dippel E, et al. Practical recommendations for therapy and monitoring of mogamulizumab patients in Germany. J Dtsch Dermatol Ges. 2025;23(3):341-354.
- Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204.
- Musiek ACM, Whittaker S, Horowitz SM, et al. Characterization and outcomes in patients with mogamulizumab-associated skin reactions in the MAVORIC trial. Eur J Cancer. 2021;156 Suppl 1:S46.

