Sézary Syndrome is an aggressive CTCL subtype,
characterized by erythroderma and abnormal (Sézary) cells in the blood1,2
Typical Sézary Syndrome presentation on the skin is highly erythrodermic. Sézary Syndrome involves a high blood tumor burden and often generalized lymphadenopathy1,2
Examples of erythroderma and hyperkeratosis in Sézary Syndrome (SS).
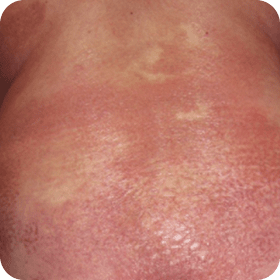
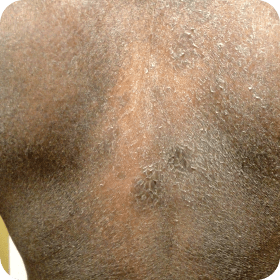

Photographs are intended as examples. Actual presentation can vary depending on individual patient factors.
- aPhotos courtesy of Joan Guitart, MD.
- bPhoto courtesy of Oleg Akilov, MD, PhD.
- CTCL=cutaneous T-cell lymphoma
Stay vigilant for signs of progression with Mycosis Fungoides and Sézary Syndrome
and progression
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133(16):1703-1714.
- Kubica AW, Pittelkow MR. Sézary syndrome. Surg Pathol Clin. 2014;7(2):191-202.
